
CEO Update – January 2026
January 20, 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen
As we reflect on 2025, I am deeply grateful for the dedication of our team and surgeon partners, whose efforts helped restore sight through 39,000+ surgeries made possible by the precious gift of donated tissue. This achievement represents not only tens of thousands of lives transformed, but also families and communities strengthened.
It was also the first full year of Cornea Tissue Addition Keratoplasty (CTAK), and the momentum has been remarkable: more than 1,500 procedures to date, 130 surgeons performing CTAK, and adoption expanding beyond the U.S. to Japan, Canada and Saudi Arabia. CTAK’s technology, using the Hersh/Greenstein/Gelles nomogram, has quickly become the most innovative solution for keratoconus treatment. If you perform CTAK , we invite you to join our CTAK User Forum for peer support, best practices and shared insights.
Looking ahead to 2026, many more countries are actively onboarding CTAK into their practice, and CorneaGen remains focused on effective training, data-driven and surgeon insights, and streamlined logistics to ensure consistent outcomes at scale. Our mission is clear: deliver reliable access to high-quality tissue and technologies that simplify care and maximize impact. None of this is possible without our partners and the broader corneal care community, so thank you for your unwavering commitment to sight restoration.
As CEO, I often review numbers, but I never lose sight of the human impact behind them. The ripple effect of our work means that not only did we collectively contribute to brighter futures for a record 39,000+ individuals last year through ocular tissue transplant, but we also made an indelible impact on their families and broader communities. Every procedure represents a story: a mother seeing her child’s first steps, a professional returning to the work they love, a neighbor re-engaging with their community. These are the moments that inspire us to keep charging forward.
Expanding Our Impact in the Gulf Coast
I’m proud to announce that last month CorneaGen formally joined forces with Lions Eye Bank of Texas at Baylor College of Medicine, marking the next step in an eight-year collaborative relationship. This acquisition strengthens our presence on the Gulf Coast and nationwide, combining their local expertise with CorneaGen’s national infrastructure to deliver greater efficiencies and high-quality ocular tissue, innovative products, and comprehensive services to more surgeons and patients. We are thrilled to have their team on board and the collective impact we will make in 2026!
Let’s Connect in 2026
We hope to connect with you again soon. Our team is currently at Hawaiian Eye and Retina Meeting and will also be at the ASCRS Annual Meeting in in Washington, D.C. We’d love the opportunity to meet up and share more about how our tissue products and services can benefit our partners.
If you’re near or visiting Orlando, South San Francisco or Seattle, we invite you to stop by our newly expanded/remodeled spaces with state-of-the-art labs designed to ensure every tissue is processed with precision and care. Last year, we nearly doubled our footprints in both Orlando and South San Francisco to continue to scale our work to meet the pressing demand of our customers.
Please reach out to tissue@corneagen.com if you’d like to set up a tour or meet up at an upcoming conference.
Reflecting on 30 Years in Eye Banking
I recently shared more about CorneaGen’s work and my 30-year eye banking journey with Ophthalmology Management for their Industry Insider feature. When asked about the most rewarding part of my career, it was hands down: the people – our team, surgeons, researchers, donors and recipients. Take a peek at the interview, which also highlights how this work became very personal to me.
Thank you for being part of this journey. Together, we’re building a future where sight restoration is accessible, outcomes are predictable, and every patient can see what’s possible. Here’s to transforming even more lives in 2026!
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -
CorneaGen Joins Forces with Lions Eye Bank of Texas
CorneaGen proudly announces its integration of Lions Eye Bank of...
READ MORE -

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
CorneaGen is excited to share that our expanded Orlando facility is now fully operational,...
READ MORE -

CEO Update – September 2025
As we transition into fall after a fast-paced summer, CorneaGen’s...
READ MORE
CorneaGen Joins Forces with Lions Eye Bank of Texas
January 15, 2026
CorneaGen proudly announces its integration of Lions Eye Bank of Texas at Baylor College of Medicine, marking the next step in an eight-year collaborative relationship.
By combining Lions Eye Bank’s deep local expertise in the Texas Gulf Coast region with CorneaGen’s nationwide infrastructure, we will create greater efficiencies and expand access to quality ocular tissue, innovative products and comprehensive services for more surgeons and their patients.
The acquisition was finalized in late December 2025, and CorneaGen and Lions Eye Bank of Texas are working closely to further integrate their organizations and teams to ensure a seamless experience for surgeons, healthcare partners and donor families. While the formal affiliation between the eye bank and Baylor College of Medicine will conclude, CorneaGen remains committed to supporting affiliated surgeons with high-quality tissue and services.
“Our partnership with the Lions Eye Bank of Texas has always been strong, and this integration marks a natural next step to accelerate our collective impact,” said Bernie Iliakis, President and CEO of CorneaGen. “By joining forces, we will strengthen our presence in Texas and across the country, enabling us to provide ocular tissue more effectively to meet the needs of patients and surgeons nationwide.”
In 2018, CorneaGen began processing and distributing tissue recovered by the Lions Eye Bank of Texas, which provided Texas surgeons greater access to ocular tissue to better meet the specific needs of their patients. In addition, their surgeon customers would be eligible to utilize CorneaGen’s extensive education and training opportunities as well as expanded offering of innovative products.
“For more than seven decades, the Lions Eye Bank of Texas has been a trusted partner in caring for the Gulf Coast community, and this transition ensures that we can continue serving patients for generations to come,” said Sumitra Khandelwal, M.D., Professor of Ophthalmology at Baylor College of Medicine. “Our partnership has shown the power of collaboration, and by formally joining CorneaGen, we’ll have greater resources to both support local eye donation and surgeons delivering sight-saving tissue to those who need it most.”
About CorneaGen
CorneaGen is a mission-driven company committed to transforming how corneal surgeons treat and care for the cornea. Based in Seattle, the company is innovating the next generation of cornea care, from new medical devices and biologics to therapeutics and interventions. CorneaGen supports corneal surgeons and their patients with a spectrum of services, including the latest in innovative products, delivery of the highest quality tissue, surgeon education, and advocacy for patient access and reimbursement policies.
Media Contact
Erika Novak, Communications Consultant
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -

CEO Update – January 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen As we...
READ MORE -

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
CorneaGen is excited to share that our expanded Orlando facility is now fully operational,...
READ MORE -

CEO Update – September 2025
As we transition into fall after a fast-paced summer, CorneaGen’s...
READ MORE
Reducing Fungal Risk in Corneal Transplants: Insights from Nicole Fram, M.D.’s Amphotericin B Research
December 10, 2025
CorneaGen’s recent discussion with Dr. Nicole Fram highlights a current question in corneal transplantation: Is amphotericin B (Ampho B) safe to add to corneal storage media? Dr. Fram’s research explores this through a large-scale retrospective review and offers valuable insights for clinicians and eye banks.
Why This Matters
Fungal infections are among the most dreaded complications in corneal transplantation. They are rare but devastating, are difficult to treat and often lead to poor outcomes. As Dr. Fram explains, “I can redo an EK or PKP. I can’t redo a fungal infection.” This underscores the importance of prevention.
Key Insights from our Interview with Dr. Fram
- Massive Data Set: The study analyzed 53,000 grafts over four years, with about half supplemented with amphotericin B.
- 10-Fold Reduction in Fungal Infection: Supplementation led to a dramatic decrease in fungal infections compared to non-supplemented tissue.
- Safety Confirmed for EK Cohorts: For DSEK and DMEK patients, there was no statistically significant increase in primary graft failure or early regraft rates when Ampho B was used.
- Mixed Results in PK Cohorts: When all patients were combined, there was a statistical difference in primary graft failure rates, suggesting the need for further analysis of PK cases.
Risk-Benefit Perspective
Surgeons weigh the risk of fungal infection against the possibility of slightly higher graft failure rates. As Dr. Fram notes, “If you’ve ever had a fungal infection in your career, it’s something you never want to see again.” This makes Ampho B supplementation a compelling option for many clinicians.
Next Steps in Research
The discussion emphasizes:
- Better Reporting Systems: Real-time outcome tracking for graft failures and regrafts.
- Prospective Studies: To confirm findings and explore optimal concentrations and formulations (pellet vs. liquid).
- Further PK Analysis: Understanding why PK cases influenced overall statistical outcomes.
CorneaGen’s Commitment
CorneaGen continues to lead innovation by being the first eye bank to provide the option of adding Ampho B to corneal preservation media. For more details on adding Ampho B to your next request or to access educational resources, visit CorneaGen.com/AmphoB or email tissue@corneagen.com.
The Bottom Line: Ampho B supplementation offers a significant reduction in fungal infection risk without compromising safety in EK procedures. While questions remain for PK cases, the evidence strongly supports its role in improving patient outcomes.
Recent Articles
-

Reshaping the Future of Keratoconus Care: Dr. Rahul Tonk on CTAK at AAO 2025
Watch the full presentation on our Education Page. At the...
READ MORE -

Why Surgeons Are Switching to the I-II Marking for DMEK and DSEK
For years, the S-stamp has been the go-to orientation marking,...
READ MORE

Reshaping the Future of Keratoconus Care: Dr. Rahul Tonk on CTAK at AAO 2025
November 19, 2025
Watch the full presentation on our Education Page.
At the American Academy of Ophthalmology (AAO 2025) meeting, corneal surgeon Dr. Rahul Tonk presented his clinical insights into CTAK (Corneal Tissue Addition for Keratoconus), a customized, tissue-based innovation from CorneaGen designed to improve visual function and corneal shape. CTAK is emerging as one of the most promising innovations for patients with keratoconus.
“Importantly, it’s customized tissue. So we’re able to customize thickness, arc length, optical zone, and so on and so forth. We’re able to apply customization by using pre-operative tomography… It gets run through CorneaGen’s nomogram…and then cut to specification,” said Dr. Tonk.
What Is CTAK? A Customized, Biologic Approach to Keratoconus Treatment
CTAK is an advanced procedure that uses gamma-irradiated, sterile, non-immunogenic corneal tissue inlays. These inlays are custom-cut based on the patient’s corneal tomography, with each graft tailored to the severity and location of the cone.
This biologic, tissue-addition technique allows surgeons to target irregular astigmatism at its source, helping normalize the corneal surface and enhance vision.
Key Advantages of CTAK for Keratoconus Patients
- Patient-ready, gamma-irradiated donor tissue that reduces immunologic risk and maintains natural corneal lamellae
- Customized laser-cut design guided by precise tomography data
- Biologic tissue-based contouring, offering a natural alternative to synthetic implants
- Minimally invasive and reversible
Unlike intracorneal ring segments or full-thickness grafts that remove or replace tissue, CTAK reshapes the cornea by adding biologic tissue, offering a more physiologic and adaptable solution.
CTAK Workflow: Streamlined Planning and Efficient Surgical Procedure
CTAK integrates smoothly into modern refractive and corneal practices. Surgeons upload tomography data through a secure platform, and CorneaGen uses a proprietary nomogram to design the custom allograft. The tissue inlay arrives ready for implantation and can be stored until the procedure (CTAK has a 2-year shelf life).
During the CTAK Procedure
- A femtosecond laser creates a precise corneal channel.
- The custom-cut tissue inlay is manually inserted into the channel.
- The tissue inlay is positioned at a superficial depth of ~200 microns, allowing significant anterior shape change while preserving structural integrity.
“It’s all a very streamlined process, very user friendly,” Dr. Tonk explained.
CTAK Clinical Outcomes: Improved Vision and Corneal Stability
Dr. Tonk’s clinical experience with CTAK for keratoconus shows encouraging improvements in:
- Uncorrected and corrected visual acuity (UCVA and BCVA)
- Corneal smoothness and regularity
- Improved tolerance for glasses or lenses
- Enhanced quality of vision
Dr. Tonk noticed that many of his patients experience reduced dependence on specialty contact lenses, while those who continue wearing them often enjoy a better fit and increased comfort.
“If we can raise [the patient’s] uncorrected and their best spectacle-corrected acuity, we’re raising the floor… and their RGP fits are going to be so much better after you flatten the cornea,” said Dr. Tonk.
Why CTAK Matters: A New Option for the Keratoconus Treatment
CTAK represents a significant advancement in corneal therapeutics, offering a personalized, minimally invasive solution for keratoconus and other ectatic disorders. Its ability to reshape the cornea using biologic tissue makes it an appealing option for patients who may not be ready, or able, to undergo more invasive procedures like a transplant.
About CTAK
CTAK is CorneaGen’s proprietary corneal contouring procedure engineered to:
- Improve corneal regularity
- Enhance corneal thickness in ectatic areas
- Boost visual performance
CTAK uses sterile, gamma-irradiated, laser-cut donor tissue inlays uniquely designed for each patient’s corneal topography.
The procedure is currently available only to trained surgeons in select regions. Patient selection and outcomes may vary depending on disease severity and surgical technique.
Recent Articles
-
Reducing Fungal Risk in Corneal Transplants: Insights from Nicole Fram, M.D.’s Amphotericin B Research
CorneaGen’s recent discussion with Dr. Nicole Fram highlights a current...
READ MORE -

Why Surgeons Are Switching to the I-II Marking for DMEK and DSEK
For years, the S-stamp has been the go-to orientation marking,...
READ MORE

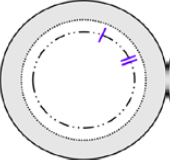
Why Surgeons Are Switching to the I-II Marking for DMEK and DSEK
November 13, 2025
For years, the S-stamp has been the go-to orientation marking, but it’s not perfect. That’s why we strongly recommend trying the I-II marking for your next surgery and here’s why.
What Makes I-II Different?
- Less Ink, Less Risk: The I-II marking uses significantly less ink, reducing potential endothelial cell damage.
- Peripheral Placement: Keeps markings out of the visual axis.
- Quick Orientation: The linear marks appear sooner during unfolding, saving time and reducing stress.
| Anterior | Posterior | Actual Posterior Image |
 |
 |
 |
Our Chief Medical Director, Matthew Giegengack, M.D., explains why he made the switch:
“I’ve been exclusively using the I-II marker for my DMEK and DSEK surgeries for several years now. The I-II mark is more peripheral, which feels better to me. The ink probably does have some endothelial toxicity, and the I-II mark is substantially smaller than the S mark.
But the biggest reason? When you’re unfolding tissue, the I-II marks come into view quicker than the S mark. Sometimes the S mark is hidden in the fold, and you can’t see it until the graft is almost fully unfolded. With I-II, I know the orientation right away, and that makes the unfolding process much easier.”
Want to Try It?
Switching is simple. Just let your customer advocate know or email tissue@corneagen.com. And if you’re looking for tips, videos, or surgical pearls, check out our resource library at corneagen.com.
Recent Articles
-
Reducing Fungal Risk in Corneal Transplants: Insights from Nicole Fram, M.D.’s Amphotericin B Research
CorneaGen’s recent discussion with Dr. Nicole Fram highlights a current...
READ MORE -

Reshaping the Future of Keratoconus Care: Dr. Rahul Tonk on CTAK at AAO 2025
Watch the full presentation on our Education Page. At the...
READ MORE

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
November 3, 2025
CorneaGen is excited to share that our expanded Orlando facility is now fully operational, marking a significant milestone in our continued growth and commitment to excellence. This new space represents more than a physical expansion; it’s a meaningful investment in our ability to support surgeons, honor donors and serve patients across Florida and beyond.
After 18 months of dedicated planning, construction and preparation, our Orlando operations have moved into a state-of-the-art facility nearly three times the size of our previous space, growing from 2,300 square feet to nearly 7,000.
This expansion enables us to process and distribute significantly more tissue, supporting the growing needs of surgeons and their patients while strengthening donor operations throughout the region. Every aspect of this growth was designed to help CorneaGen better deliver on our mission to provide the highest quality donor tissue, unparalleled customer service and superior products to ophthalmologists.
Designed for Excellence and Efficiency
The centerpiece of the new facility is a modular processing laboratory featuring four dedicated bays, which is a step up from the three areas in the former location. Combined with an optimized workflow layout, this advanced configuration enhances efficiency and consistency, allowing us to reliably deliver quality tissue to our surgeons.
Beyond functionality, we’ve created a space that reflects our commitment to excellence. With more natural light, modern design and an uplifting work environment, the facility embodies the same care and precision that define every cornea we process.
Strengthening Our Community and Partnerships
Our new facility isn’t just a workspace, it’s a destination for connection. With dedicated areas to welcome donor partners, surgeons, hospitals and community stakeholders, we’re excited to create opportunities for collaboration and celebration. The moment you walk into the space, the real impact of our work to honor the gift of sight will be brought to life with large visual displays that highlight donor and recipient stories – allowing visitors to fully immerse into our mission.
This milestone reflects the passion, collaboration and expertise of the CorneaGen team, all of whom bring an unwavering dedication to quality and excellence every day. From planning and design to technical execution and operational readiness, their collective effort made this achievement possible and ensured a seamless transition into our new space. While this achievement was made possible by the dedication of countless individuals, we’d like to recognize several key contributors, including Andre Clark, Lindsey Elbanhawy, Kevin Gibson, DiAnn McCormack, Andrew Beal, and Jessica Moore, along with the entire Orlando team, whose focus, collaboration, and determination brought this vision to life.
Serving Surgeons Coast to Coast
With the Orlando expansion joining our recently upgraded San Francisco, Seattle and Winston-Salem laboratories, CorneaGen now has a stronger foundation to serve surgeons from coast to coast, reinforcing our ongoing commitment to quality, innovation and partnership that define our mission.
As we continue to grow, one thing remains constant: our unwavering commitment to the surgeons and patients we serve. Together, we’re creating a brighter future, one cornea at a time.
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -

CEO Update – January 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen As we...
READ MORE -
CorneaGen Joins Forces with Lions Eye Bank of Texas
CorneaGen proudly announces its integration of Lions Eye Bank of...
READ MORE -

CEO Update – September 2025
As we transition into fall after a fast-paced summer, CorneaGen’s...
READ MORE

CEO Update – September 2025
September 8, 2025
As we transition into fall after a fast-paced summer, CorneaGen’s momentum is only accelerating, especially with the progress we’re making with Cornea Tissue Addition Keratoplasty (CTAK). This innovative procedure continues to gain recognition from surgeons, patients and the media, and I’m proud to share several exciting milestones:
- 1,000 CTAK procedures: In a few weeks, we will cross this important threshold less than 18 months after launch, reflecting both rapid surgeon adoption and strong patient outcomes.
- Growing surgeon adoption: We’re about to surpass 100 trained surgeons performing CTAK, with many inquiries still coming in.
- Expanded compatibility: As announced in July, CTAK is now fully compatible with Ziemer USA Inc.’s GALILEI Diagnostic Platform and FEMTO LDV Z8 Femtosecond Laser Platform, broadening access for surgeons worldwide.
- International growth: CTAK procedures are expanding outside the U.S., beginning in Saudi Arabia, Canada and Japan with more countries on the horizon. If you’re attending ESCRS 2025, visit us at booth #B5-621 to see how CTAK can become part of your practice.
- COMING SOON – Online user group: In response to surgeon demand, we will launch the first CTAK user group at AAO, giving surgeons a dedicated online forum to connect, share experiences and exchange case management tips.
If you haven’t yet taken the leap, our team is poised and ready to help you get started with CTAK today.
Continuing to Deliver High-Quality, Safe Transplant Tissue
As for our transplant tissue side of the organization, we recently had a fantastic conversation with Nicole Fram, M.D. discussing her clinical research with Matt Giegengack, M.D. on the safety and efficacy of supplementation of corneal storage media with Amphotericin B. CorneaGen was proud to lead the charge back in 2018 as the first U.S. eye bank to offer Amphotericin B as an additive to corneal storage media.
We did it with one goal in mind: patient safety. As Dr. Fram says, “The most dreaded complication of any corneal transplantation…is a fungal infection.” If you’re interested in learning more, Dr. Fram will be presenting their research at the Cornea and Eye Banking Forum on October 17, just before AAO.
We’re also excited to share that our new Orlando eye bank facility is now operational. With double the capacity, we can better meet growing global demand and continue setting the standard for corneal recovery and DSAEK/DMEK processing quality in the U.S. and abroad.
CTAK Capturing Media Attention
Our work has recently been highlighted by several leading outlets, including Media Mice, IDocSocial and coverage of our expanded partnership with Ziemer USA Inc. Take a peek:
- Media Mice Feature – highlighting CTAK as a new era in keratoconus treatment
- IDocSocial Interview – sharing insights on innovation and patient impact
- Ziemer USA Inc. Partnership – focusing on expanding CTAK access
Reflecting on What Drives Us
At CorneaGen, we end every staff meeting by reading letters or stories from donor families and recipients as a reminder of why we do this work: to make a lasting impact on our communities, neighbors, families and friends.
One such story came from Lisa, who shared: “I go outside every night to look at the stars and marvel at their beauty. I am so much more aware of the importance of sight and not taking it for granted. I give thanks every day for the donation that changed my life and enabled me to continue doing the things I love.”
Her words remind us that every innovation, every partnership and every procedure comes down to this: the gift of sight. To our partners, surgeons and teams – thank you for helping donor families find hope in their healing and recipients see the world through new eyes.
Onward, together!
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -

CEO Update – January 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen As we...
READ MORE -
CorneaGen Joins Forces with Lions Eye Bank of Texas
CorneaGen proudly announces its integration of Lions Eye Bank of...
READ MORE -

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
CorneaGen is excited to share that our expanded Orlando facility is now fully operational,...
READ MORE
CTAK Expands Surgeon Access: Procedure Now Compatible with Ziemer GALILEI platform
July 24, 2025
CTAK Expands Surgeon Access: Procedure Now Compatible with Ziemer GALILEI platform
Corneal Tissue Addition for Keratoplasty (CTAK), the groundbreaking solution for contouring keratoconic eyes, has taken a major step forward in accessibility with the recent integration with Ziemer GALILEI technology. Until now, CTAK’s compatibility was limited to ophthalmology clinics using the OCULUS Pentacam® to assess the surface of a patient’s eye – specifically the severity and location of the cone shape, the hallmark sign of keratoconus.
“The integration of CTAK with the Ziemer GALILEI diagnostic platform marks a significant step forward in the precision and personalization of keratoconus treatment,” said Dr. Peter S. Hersh, MD, originator of CTAK. “By combining high-resolution corneal tomography with laser-guided stromal inlay preparation, we can deliver an anatomically optimized and patient-specific approach to corneal reshaping. Such technological innovation moves us to safer, more predictable, and meaningful improvements in visual function for patients with keratoconus.”
After nearly a decade of development, CTAK was commercially launched by CorneaGen in 2024 and continues to gain momentum in the industry, helping surgeons transcend the traditional standards of care in treating keratoconus. CorneaGen is the exclusive provider of the custom, patient-ready tissue inlays for the CTAK procedure.
“At CorneaGen, our mission goes beyond innovating affordable technologies and treatments – it’s about expanding access to innovations for patients around the world,” said Bernie Iliakis, CEO of CorneaGen. “Our partnership with Ziemer and the integration of their advanced GALILEI imaging systems mark a pivotal step in this journey.”
The GALILEI CTAK Software is now available and requires a system update to surgeon’s existing GALILEI platform. David Bragg, President of Ziemer USA, said, “We are thrilled to complete our product offering for CTAK patients. With the addition of the Galilei CTAK software, in combination with our FEMTO Z8 LDV femtosecond laser platform, we can now provide surgeons and patients with a fully integrated surgical solution aimed at restoring vision and improving outcomes.”
In the coming months, CorneaGen will partner with a cohort of surgeons using the Ziemer GALILEI diagnostic platform to integrate the procedure into their practice, making high-quality, personalized keratoconus treatment more widely available.
In addition, CorneaGen will continue to make progress on the expansion of CTAK’s global footprint. With nearly 20% of ASCRS wet lab participants practicing outside the U.S., there is significant opportunity for continued global growth.
By making CTAK platform-agnostic and expanding globally, CorneaGen expects increased adoption of CTAK across academic centers, ambulatory surgery centers, and private practices worldwide. As more surgeons gain access to its capabilities, the future of keratoconus treatment looks increasingly precise, personalized, and patient-centered.
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -

CEO Update – January 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen As we...
READ MORE -
CorneaGen Joins Forces with Lions Eye Bank of Texas
CorneaGen proudly announces its integration of Lions Eye Bank of...
READ MORE -

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
CorneaGen is excited to share that our expanded Orlando facility is now fully operational,...
READ MORE

Bernie’s Quarterly Update – November 2024
November 13, 2024
A message from Bernie Iliakis, President & CEO of CorneaGen
We’re nearing the end of another year, having just wrapped AAO 2024—things are busy as always at CorneaGen, so I’m excited to share a few third quarter highlights and upcoming opportunities.
Industry events and participation
CorneaGen attended ESCRS in September and AAO in October, meeting with partners, sharing innovations, and showcasing our solutions. Much of the talk was around the hot topic of Corneal Allogenic Intrastromal Ring Segments (CAIRS) and our customized solution, CTAK.
Corneal Tissue Addition Keratoplasty (CTAK) continues to have immense interest. It was our headliner for AAO, with 111 surgeons trained in wet labs throughout the course of the event. We have more than 300 surgeons interested in the solution, with nearly 165 on board and ready to submit patients. Dr. Peter Hersh (Teaneck, NJ), creator of CTAK, and Dr. Brandon Ayres (Wills Eye Hospital, Philadelphia) joined us at AAO to present on CTAK benefits for patients, sharing how the procedure can reshape the cornea and return sight. This innovative solution to corneal contouring represents a true treatment advancement for keratoconic eyes. We continue to be very excited about the ongoing work that is changing the lives of those affected by keratoconus and maximizing the gift of eye donation.
Also, at AAO, we were proud to highlight our EndoSerter-PL solution, demonstrating how it redefines DSEK. Dr. Yassine Daoud (Wilmer Eye Institute, Baltimore) led this discussion in the CorneaGen booth, presenting the benefits of and demonstrating pre-loaded DSEK.

We’re seeing growing international interest in CorneaGen solutions, and the work we’re doing with partners is infinitely rewarding. From the advancement of CTAK through collaborations with CTAK LLC, Ziemer, and Corza to the work with Iantrek Inc., whereby we assemble, package, and distribute its CycloPen, these partnerships make industry innovation possible.
I encourage all interested in learning more about CTAK to join me on Thursday, November 20th as I host a webinar on CTAK, presented by Dr. David Hardten (Minnesota Eye Consultants) and Dr. Elizabeth Davis (Minnesota Eye Consultants). Together, these doctors have performed over 100 CTAK procedures and they are ready to share their surgical pearls! You can register here.
Industry headwinds and developments
This year, the FDA has placed an increasing focus on tissue/eye donors with a notation of sepsis in their medical record. This is likely due to two recent tuberculosis infections that were linked to bone grafts. This has led to some eye banks being cited by the FDA for not determining donors as ineligible for transplant if there is documentation of sepsis in the medical record.
This can and already has significantly impacted the supply of ocular donor tissue for transplant in the US and abroad. It is possible final FDA guidance will require ruling out any donor with a notation of sepsis during their hospital stay. However, it is important to point out infectious disease consultants, who review a potential donor’s medical chart, as well as a Medical Director or Medical Director Designee, have always been part of making the final determination on eligibility and the track record of safety in corneal transplants is extremely high. We’re expecting additional FDA guidance soon, so more to come.
At CorneaGen, we’re well positioned and continue to maximize the gift, so we can continue to serve surgeons and patients with safe/quality tissue.
Industry conversations and discussion
I want to highlight CAKE Issue 24 – The Cornea Issue, which included CorneaGen commentary on the important role for-profits play in the eye banking industry. As you know, CorneaGen is very proud of the work we and other for-profit organizations have done to advance cornea care for the good of patients, surgeons, and the industry. We know our significant innovations (e.g. cell therapy) are possible only through a for-profit model, investor capital, and industry partnerships, as evidenced by the next-generation solutions and partnerships we’re talking about here in this message. I encourage you to read the article, starting on page 33, and know that CorneaGen remains committed to serving patients affected by corneal disease.
I close on a note of appreciation to everyone involved in supporting CorneaGen’s mission, and I look forward to finishing another year strong in 2024.
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -

CEO Update – January 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen As we...
READ MORE -
CorneaGen Joins Forces with Lions Eye Bank of Texas
CorneaGen proudly announces its integration of Lions Eye Bank of...
READ MORE -

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
CorneaGen is excited to share that our expanded Orlando facility is now fully operational,...
READ MORE

Bernie’s Quarterly Update – July 2024
July 30, 2024
A message from Bernie Iliakis, President & CEO of CorneaGen
As we close the second quarter of 2024, we’re incredibly proud at CorneaGen to have launched commercial availability of Corneal Tissue Addition for Keratoplasty (CTAK). An innovative solution to corneal contouring, CTAK is a true treatment advancement for keratoconic eyes—providing patient ready, gamma-irradiated sterile, non-immunogenic corneal tissue segments. We couldn’t be more excited about this new opportunity to change the lives of those affected by keratoconus, while also honoring and making the most of each corneal donation.
And we don’t do any of this alone—with ongoing innovations brought to the market by strong partnerships, I’m pleased to share more about what CorneaGen has achieved in the last few months.
Innovations in corneal care
CTAK co-inventors include Drs. Peter Hersh, Steven Greenstein, and John Gelles. More than 150 successful surgeries have been completed to date, and we have 30 active surgeons using the solution. Just one recent testimonial includes:
“Following a CTAK procedure, patients can experience a ‘wow’ effect with their vision. We have seen immediate impressive improvements in best corrected and uncorrected vision. This immediate improvement at the first follow-up is an exciting experience for those dealing with Keratoconus.” – Dr. William Wiley, Medical Director, Cleveland Eye Clinic Division of Midwest Vision Partners
There’s been a lot of focus around corneal allogenic intrastromal ring segments (CAIRS) in the last few years and even growing international interest in a solution such as CTAK. We want to encourage everyone interested to reach out, as we continue delivering CTAK to the market, and don’t miss the webinar we’re hosting on August 6th at 6pm Pacific, highlighting the solution.
Partnerships and philanthropy
Organizational partnerships that led to the creation of CTAK include CTAK LLC and Ziemer, and we have many others. CorneaGen has a long history with Geuder AG, as the distributor of their glass cannula used for DMEK surgery. We’re continuing to grow in the glaucoma space with collaborations like Iantrek Inc., whereby we assemble, package, and distribute the newly launched CycloPen, which utilizes scleral tissue for microinvasive glaucoma surgery (MIGS). CorneaGen’s collaborations to advance treatments, as well as efforts to provide sterile grafts for glaucoma and cornea surgeries through our Long-Term Tissue program, continue to be world class in the industry.
And I wanted to highlight CorneaGen’s Jared Young, our VP of Sales and Marketing, on his co-founding of the Trillium Vision Foundation. As this organization helps restore vision globally through a community of people and partners, CorneaGen is proud to support their philanthropic work. Watch the link below for coverage of their most recent trip to San Salvador.
https://eyewire.news/tv/trillium-vision-foundation.
Growth and expansion
In closing, I also wanted to make note of CorneaGen’s growing presence in California and Florida. Announced earlier this year, CorneaGen expanded eye recovery and processing services of donor tissue for facilities throughout California. We are in the midst of facility expansion in the Bay Area and Orlando, growing our team in both locations. We close a strong and eventful first half of 2024 with significant impact in the ophthalmic space.
Thank you as always to our surgeons and partners for helping support CorneaGen’s mission. I look forward to connecting on our progress next quarter.
Recent Articles
-

Supply, Shipping and Logistic Challenges
Due to the worsening situation in the Middle East, airspace...
READ MORE -

CEO Update – January 2026
A Message from Bernie Iliakis, President & CEO of CorneaGen As we...
READ MORE -
CorneaGen Joins Forces with Lions Eye Bank of Texas
CorneaGen proudly announces its integration of Lions Eye Bank of...
READ MORE -

Growing for Greater Impact: CorneaGen Expands in Orlando to Better Serve Surgeons and Patients
CorneaGen is excited to share that our expanded Orlando facility is now fully operational,...
READ MORE
