DSAEK
Descemet’s Stripping Automated Endothelial Keratoplasty graft thickness specification are available in micron ranges, including Nano-Thin™ <60 microns, and Ultra-Thin <100 microns.
READ MORE
Introducing the revolutionary EndoSerter®-PL. This exclusive, FDA cleared, single-use graft insertion device is pre‑loaded with expertly processed Nano‑Thin™ or Ultra-Thin DSEK tissue.
Schedule a ConsultationDiscuss with your Surgical Product Specialist today to schedule your in-service consultation and experience DSEK Redefined.
The EndoSerter-PL is an FDA cleared, single-use graft insertion device pre‑loaded with expertly processed Nano‑Thin™ or Ultra-Thin DSEK tissue. The following video provides a detailed overview of how the EndoSerter-PL works, along with step-by-step instructions on using the EndoSerter-PL. Please take a moment to watch the video and then give our office a call to schedule an in-service: (844) 526-7632
This FDA cleared, single-use graft insertion device is pre‑loaded with expertly processed Nano‑Thin™ or Ultra-Thin DSEK tissue.
DSEK REDEFINED


Innovations in Corneal Endothelial Transplantation
Edward Holland, M.D. presents his experience of working with CorneaGen's...
WATCH MORE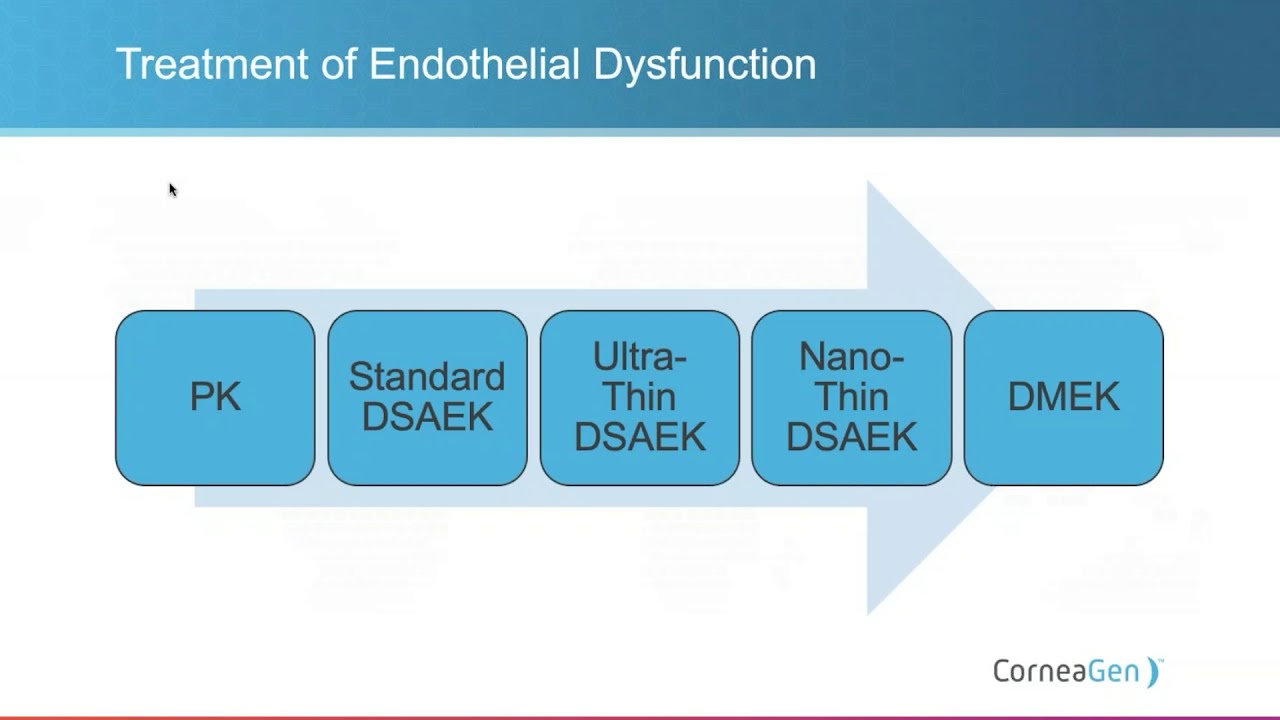
The Eye Bank Difference
Watch Dr. Matt Giegengack as he presents an informative educational...
WATCH MORE
Using the EndoSerter® to Insert Nano-Thin™, the Thinnest DSEK Tissue
CorneaGen invites you to view Dr. Zaina Al-Mohtaseb’s 2019 AAO...
WATCH MORE
EndoSerter® for DSEK
Watch Dr. Julie Schallhorn as she demonstrates her surgical techniques...
WATCH MORE
How Thin is Nano-Thin? Discover the Thinnest DSAEK Yet.
Watch this free educational webinar as Dr. Edward J. Holland...
WATCH MORE
If you need assistance with reimbursement of corneal tissue, CorneaGen is pleased to provide its customers with reimbursement and medical claims assistance to help guide you in billing corneal tissue to your local Medicare carrier and other commercial insurance carriers.
If you have had any complications occur in your recipients, we ask you please file a report with us so that we may comply with EBAA and FDA regulations.
CorneaGen’s labs maintain the highest levels of quality through review by the U.S. Food and Drug Administration. For more information, please contact our Quality Assurance and Regulatory Affairs Department at (877) 682-8502.