Geuder Pre-Loaded Glass Cannula for DMEK
The newly optimized Geuder Pre-Loaded Glass Cannula for DMEK is a single-use transportation cartridge system, pre-loaded with DMEK tissue pre-punched to your specified diameter, stained and with orientation markings.
Schedule a ConsultationDetails & Specifications
In-Servicing
Your CorneaGen Surgical Product Specialist is available to help ease your transition to the Geuder Pre-Loaded Glass Cannula for DMEK. If you are a surgeon who would like to schedule an in-service for your next DMEK, please watch the instructional video below. Then contact us to schedule your in-service by calling at (844) 526-7632.
Please note, an in-service is required prior to initial use of the Geuder Pre-Loaded Glass Cannula.
The Geuder Pre-Loaded Glass Cannula for DMEK is a disposable, single-use transportation cartridge system, pre‑loaded with DMEK tissue and ready to insert. The following video provides a detailed overview of how the Geuder Pre-Loaded Glass Cannula works, along with step-by-step instructions on using the Cannula. Take a moment to watch the video and give our office a call to schedule an in-service: (844) 526-7632.
Product Info
Simplifies DMEK
DMEK allograft is prepared to your specified diameter, pre-punched, stained and with an s-stamp.
Ready-to-Insert
Comes pre-loaded with DMEK tissue, saving you time in the O.R.
Consistent Incision Size
The 2.4 mm incision size allows for gentle and controlled injection into the anterior chamber.
Surgical Video
Return Policy
Various circumstances arise that require a customer to return tissue. For such cases, please find our return policy on our Billing Terms and Conditions.


Educational Resources
To advance the transfer of knowledge within the cornea ecosystem, we offer world-class surgeon educational offerings.

Part 3 – Advanced DMEK: Complicated Cases – A Roundtable Discussion
Dr. Nicole Fram, Dr. Terry Kim and Dr. Elizabeth Yeu...
WATCH MORE
Part Two – Advanced DMEK : A Roundtable Discussion on Complicated Cases
Our expert panel discuss their complicated cases and share the...
WATCH MORE
Handling of the Geuder Pre-Loaded Glass Cannula for DMEK
Schedule time with a Surgical Product Specialist to learn more!
WATCH MORE
Advanced DMEK: A Roundtable Discussion on Complicated Cases
Dr. Nicole Fram, Dr. Elizabeth Yeu and Dr. Terry Kim...
WATCH MORE
DMEK Using the Innovative Geuder Pre-Loaded Glass Cannula
Michael Banitt, M.D. presents his experience with DMEK using the...
WATCH MORE
Advanced DMEK: Complicated Cases
In this recorded webinar, our panel of experienced DMEK surgeons,...
WATCH MORE
DMEK: Fast, Simple, and Reliable with the Pre-Loaded Geuder Cannula
Marjan Farid, M.D. presents on her experiences with DMEK with...
WATCH MORE
DMEK: Fast, Simple, and Reliable with the Pre-Loaded Geuder Cannula
Charles Lin, M.D. presents on his experiences with DMEK with...
WATCH MORE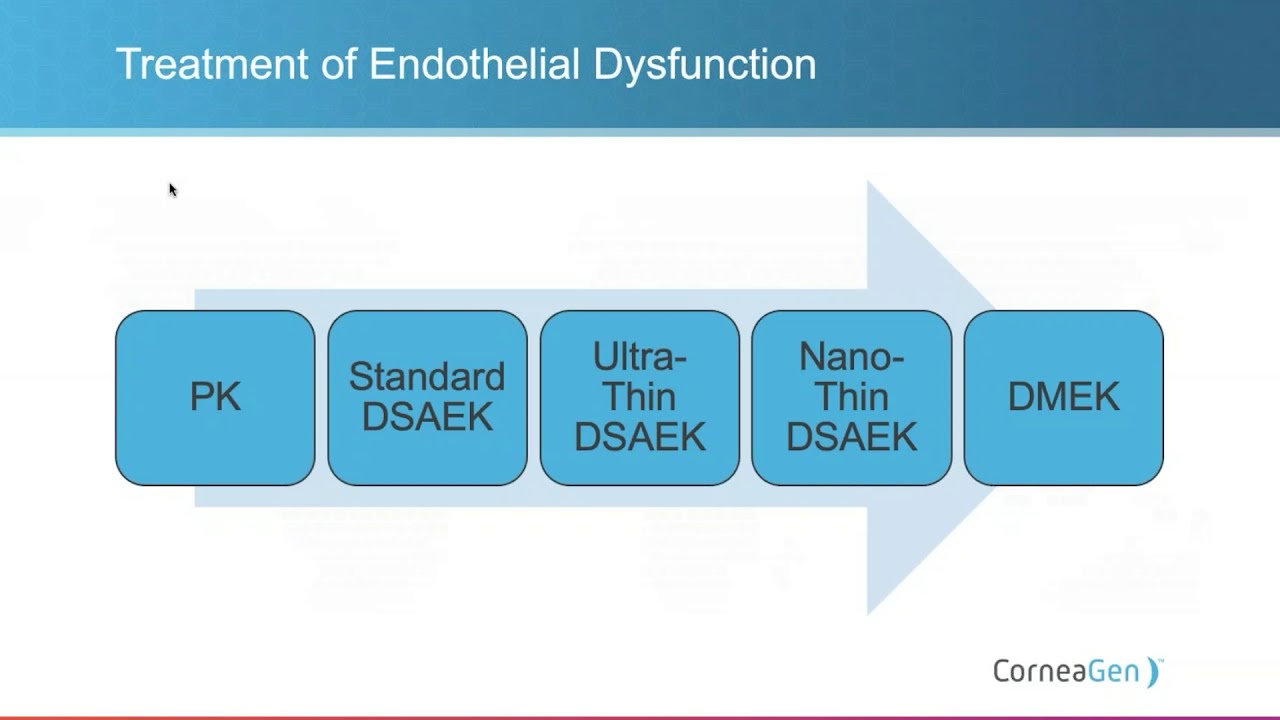
The Eye Bank Difference
Watch Dr. Matt Giegengack as he presents an informative educational...
WATCH MORERelated Products

Corneal Tissue Reimbursement
If you need assistance with reimbursement of corneal tissue, CorneaGen is pleased to provide its customers with reimbursement and medical claims assistance to help guide you in billing corneal tissue to your local Medicare carrier and other commercial insurance carriers.
Adverse Reaction Reporting
If you have had any complications occur in your recipients, we ask you please file a report with us so that we may comply with EBAA and FDA regulations.
Regulatory Information
CorneaGen’s labs maintain the highest levels of quality through review by the U.S. Food and Drug Administration. For more information, please contact our Quality Assurance and Regulatory Affairs Department at (877) 682-8502.




