CTAK International
Corneal Tissue Addition for Keratoplasty (CTAK) is expanding internationally. Contact us to get started.
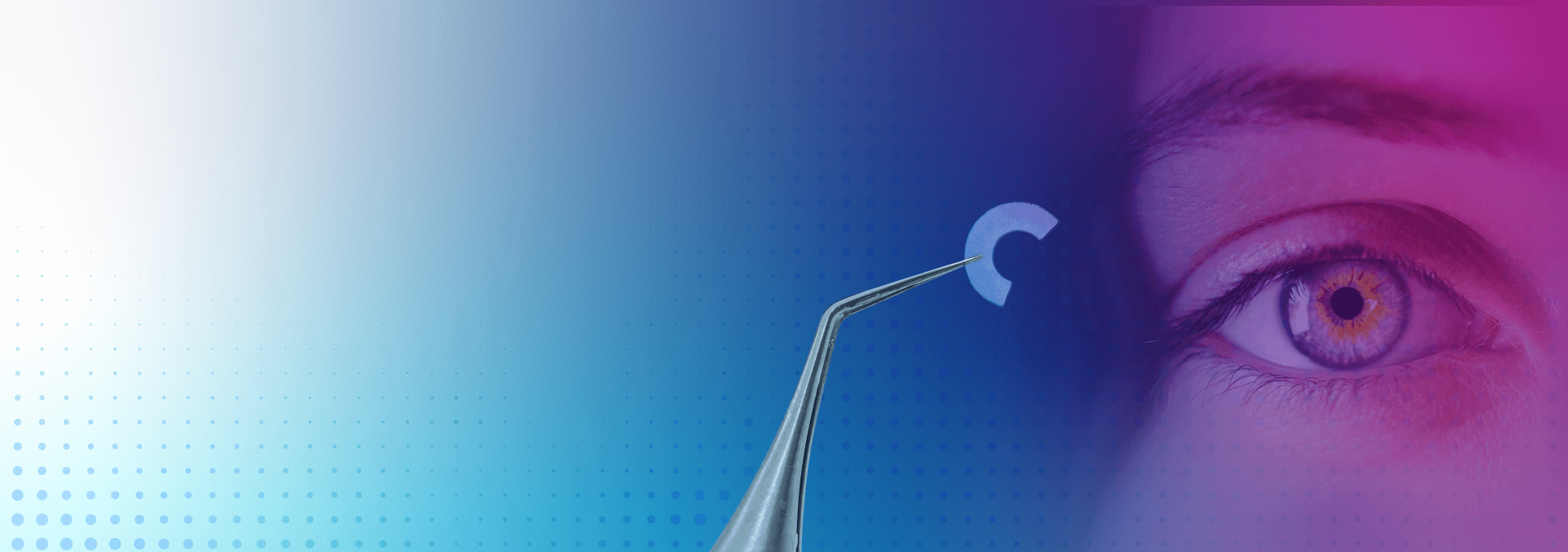
This innovative procedure offers an advanced solution for contouring keratoconic eyes and is designed to significantly improve sight for patients in need. Using CorneaGen’s Custom Tissue Inlays, CTAK provides patient-ready, gamma-irradiated sterile, non‑immunogenic donated corneal tissue inlays that are laser-cut and customized to patients’ specific severity and cone location.
Get Started with CTAK
About CTAK
CTAK is the latest advancement in keratoconus treatment, focused on accuracy and precision for both patients and surgeons. This innovative procedure involves the use custom tissue inlays, designed to optimize corneal flattening, improve visual acuity, and add thickness to the cornea.
Using a Ziemer Neo Z8 Femtosecond Laser, CorneaGen processes tissue to the precise parameters for each patient. The tissue is sterilized, packaged and shipped with custom surgical plan options.
CTAK is designed to improve visual outcomes, greater surgical precision, and faster recovery times.
- Precision and accuracy of a Ziemer Z8 laser-cut custom tissue inlay
- Biocompatible and fully customizable
- Clinical study showed no rejection of tissue post-transplant
- Custom surgical options detail inlay placement and sizing.
- Topography flattening and visual acuity improvement
- Gamma-irradiation adds rigidity to the inlay and stability for the surgeon
Moderate keratoconus: 50D-75D.
Patients with ectasia need more care and can schedule a consultation.
- Moderate sized cones: ≤4.0mm in width
- No cone which extends outside of the central 6mm optical zone (~3.0mm from the center of the pupil)
- No scarring in the visual axis
Greenstein, Steven A. MD; Yu, Austin S. BA; Gelles, John D. OD; Eshraghi, Hamoon MD; Hersh, Peter S. MD.
Journal of Cataract & Refractive Surgery 49(7):p 740-746, July 2023. | DOI: 10.1097/j.jcrs.0000000000001187
We’re excited to help you take the first step toward offering this innovative procedure by offering a short 15-20 minute online training course for surgeons interested in offering CTAK in their practice.
The online CTAK training will:
- Introduce you to CTAK and its procedural requirements
- Provide insights into patient selection and surgical planning
- Offer step-by-step instructions to prepare you for hands-on practice
Visit CorneaGen.com/CTAKtraining and start today!

Educational Resources
CorneaGen offers world-class surgeon educational offerings including Webinars, Surgical footage, and pearls videos.
We partner with leading surgeons and experts to share insights and pearls from their successes and experiences.
Learn something today!

CTAK for Keratoconus: Dr. Rahul Tonk’s Full Presentation at AAO 2025
Learn about CTAK (Corneal Tissue Addition for Keratoconus) in this...
WATCH MORE
CTAK: Surgical and Clinical Pearls Booth Talk at ASCRS 2025
CTAK creator, Peter Hersh, M.D., is joined by Elizabeth Davis,...
WATCH MOREGet Started with CTAK Today

Adverse Reaction Reporting
If you have had any complications occur in your recipients, we ask you please file a report with us so that we may comply with EBAA and FDA regulations.
Regulatory Information
CorneaGen’s labs maintain the highest levels of quality through review by the U.S. Food and Drug Administration. For more information, please contact our Quality Assurance and Regulatory Affairs Department at (877) 682-8502.







